
SOLUTION FOR THE HARDEST-TO-RECYCLE WASTE ON THE PLANT
Currently, disposable lunch boxes, spoons, forks, drinking cups, or egg cartons are usually made of polystyrene (PS) plastic.
GRAPHENE: THE SUPER MATERIAL FORMULATED FROM PLASTIC
COOLING PAINT
PLASTIC FOAM RECYCLING
FLEXIBLE GLASSES - INSPIRATION FROM SEASHELLS
SOLUTION FOR THE HARDEST-TO-RECYCLE WASTE ON THE PLANT
Currently, disposable lunch boxes, spoons, forks, drinking cups, or egg cartons are usually made of polystyrene (PS) plastic. With many advantages such as low cost, good insulation, water resistance, and resistance to many acids and bases, this plastic is widely used in daily life.
Consequently, they account for more than a third of all plastic waste globally. Since it's 95% air, collecting and recycling polystyrene can make it more expensive than making polystyrene yourself from scratch.
Fortunately, the Journal of the American Chemical Society recently reported that scientists have found a way to recycle polystyrene plastic into a more economically valuable product, benzoic acid.
Benzoic acid can be sold for twice as much as polystyrene. The benefits now outweigh the costs of recycling plastic, as benzoic acid is an important precursor used in the chemical and biological industries and is used in medicine, perfumery, food preservatives, and many other applications.

Photo: Product made from PS plastic
>>>Click for the best RUBBER INDUSTRY CHEMICALS on market at present<<<
Using sunlight to recycle the "hardest" plastic on the planet
The new research was carried out by scientists from Cornell University, USA. In it, they used an iron chloride catalyst to dissolve polystyrene resin. After that, the work is very simple. Just leave the mixture under a sunny window and in an oxygenated environment. The reaction that turns polystyrene into benzoic acid will automatically occur.
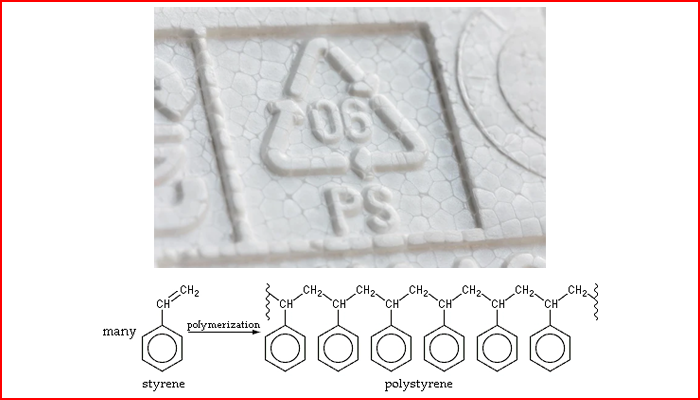
Photo: The chemical formula of polystyrene resin
At the atomic level, polystyrene is a polymeric plastic made up of hundreds of monomer molecules arranged in long chains through complex processes, said Erin Stache, Associate Professor of Chemistry and Chemical Biology at Cornell University said. The task now is to break the bonds of the monomer before turning it into another compound.
Current polymer recycling methods all require heating to melt the plastic before processing. That means recycling - a seemingly eco-friendly activity - uses fossil fuels, often coal, to melt the plastic.
"The advantage of using light in our method is that you can control the chemistry well based on some of the catalysts we've developed. This method harnesses energy from white light. If we can use sunlight to promote recycling, that's a double-hit arrow", said Stache.

Photo: Stache's experiments with lamps that simulate sunlight and balloons that blow a stream of oxygen.
Stache's experiments with lamps simulate sunlight and balloons that blow a stream of oxygen.
In his experiment, he saw the polystyrene plastic sample decompose in just 20 hours allowing the team to obtain 23% benzoic acid. Then, Stache switched to recycling common polystyrene plastic waste, from styrofoam boxes to coffee cup lids.
"They all decompose very well", said Stache. White styrofoam cans can convert to benzoic acid more efficiently than black coffee cup lids because white absorbs sunlight better.
In addition, the reaction can also work in the condition that there are still impurities such as leftovers, or additives attached to the plastic. "These results show that our system can efficiently degrade commercial polystyrene samples, even in the presence of composite and insoluble materials", said Stache.
Benzoic acid is obtained from recycled polystyrene plastic.
Catalyst shortens plastic degradation time from 10 years to 24 hours
Using the same catalyst, another team from the University of Texas aimed to degrade PET (polyethylene terephthalate polymer) plastic. This is a type of plastic commonly used to make clothes, food packaging, and especially liquid bottles, including drinking water.
PET plastic accounts for about 12% of global plastic waste. The amount is lower than polystyrene, but considering the amount when there are currently 6.3 billion tons of plastic waste being stored on Earth, that would still be a huge number. The world has about 8.3 billion tons of plastic, equivalent to 822,000 times the Eiffel Tower or 1 billion elephants. 6.3 billion tons of that is waste and only 9% of plastic is recycled.
To solve the problem, the Texas team used artificial intelligence to screen the enzyme designs for a biological catalyst called FAST-PETase. In which, F stands for "functional", A is "active", S stands for "stable", T is "tolerant", ase is an enzyme base.
This catalyst can cut PET plastic into its monomer molecules through a molecular reduction process. The team then demonstrated they could assemble these monomer molecules into polymers.
The process occurs at temperatures below 50 degrees Celsius, which means it can also happen on a mildly sunny day. You just dump FAST-PETase down a plastic landfill and let the enzyme do its job. Experiments show that FAST-PETase can degrade PET plastic within 24 hours instead of the process that takes decades in nature.
And because FAST-PETase is a biocatalyst, it is also environmentally friendly. "Our work demonstrates the power of science when it comes to bringing together interdisciplinary disciplines, from synthetic biology to chemical engineering and artificial intelligence", said biochemist Andrew Ellington, a researcher at the University of Texas.
Source: Nature magazine.
>> Click HERE to read our UPDATED NEWS<<
Contact
MEGA VIETNAM
Office address: Floor 2-A2-IA20, Nam Thang Long Urban Area, Pham Van Dong Street,
Dong Ngac Ward, Bac Tu Liem District, Hanoi City, Vietnam
Email: contact@megavietnam.vn
Tel: (+84) 24 375 89089; Fax: (+84) 24 375 89 098
Website: megavietnam.vn
Hotline: 1800.577.728 Zalo: 0971.023.523





